Many Canadians have been trying to learn what they can about Canada’s Assisted Human Reproduction Act. Many lawyers have been calling for regulatory change and an update to the laws.
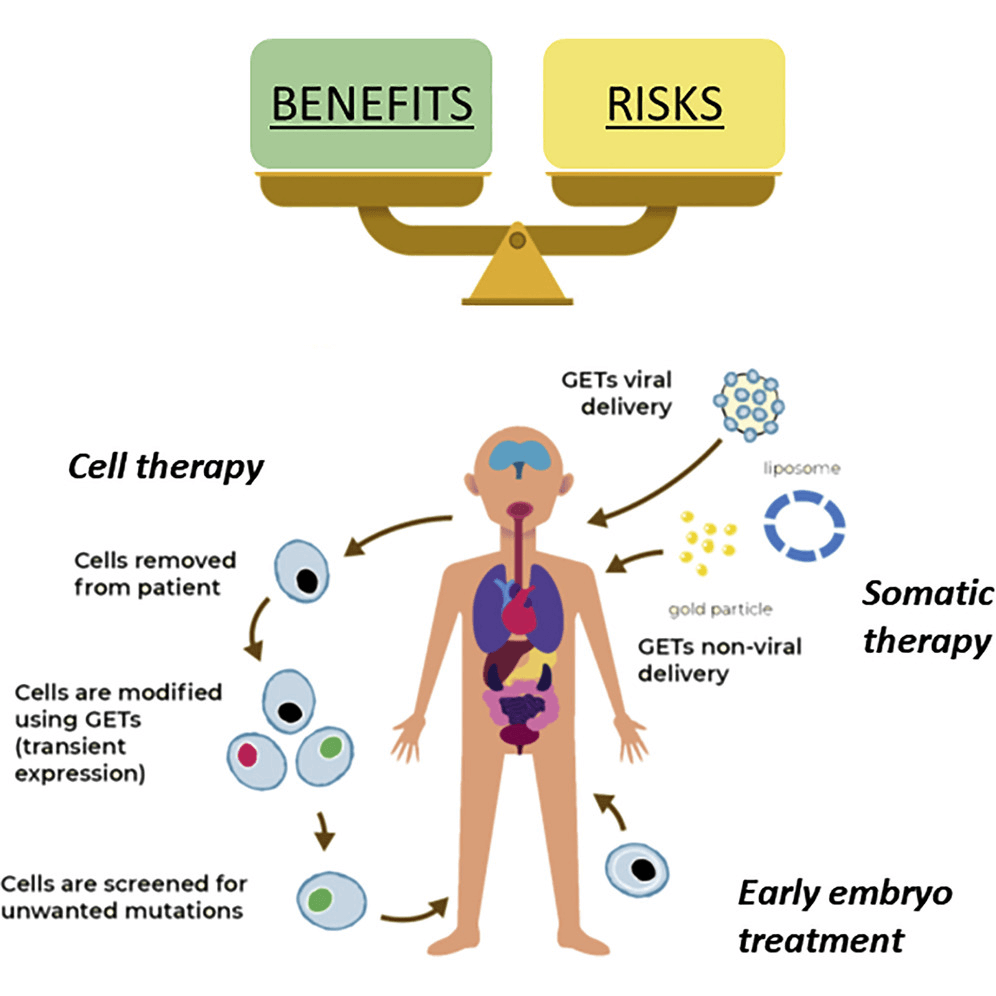
The Act concerns itself with vitro research and gene editing. We found the most simple description of what gene editing is below, also called Genome Editing.
Photo: Genome Editing
Many want mitochondrial replacement therapy to be legalized in Canada. This will help more infertile women have babies. You can learn more about MRT on the National Library of Medicine website.
In or around 2019 the Assisted Human Reproduction Canada program was cancelled.